Zonulin and Leaky Gut: What you Need to Know

Contents
Autoimmune leaky gut connection not conclusive
However, as far as the science goes these associations are currently unsupported, with the quote below from a recent review giving the most concise statement:Although changes in barrier function have been described in several gastrointestinal disorders, their primacy remains to be defined. At present, few studies support efficacy for an intervention that improves barrier function in altering the natural history of a disease process. Quigley EM, Curr Opin Gastroenterol. 2016 2I’ve bolded what I would consider to be the key statement. So whilst the evidence for a “leaky gut” as a driver of certain diseases is weak, there are numerous disorders which can both drive and result from its occurrence.
Structure and function of the gut
Before we go on, let’s have a look at how the lining of the gut functions and how it could become “leaky.” The inside of your gut is lined with a single layer of epithelial cells which make up the mucosal barrier. These cells are highly specialized having to allow absorption of all nutrients the body requires, whilst also stopping harmful compounds or invading microorganisms from passing across the mucosal barrier into the blood stream. To do this, epithelial cells have two major protective features;- They secrete mucus into the gut which acts to trap harmful microorganisms before they reach the epithelium.
- Secondly, they form an impermeable wall by linking together with so called “tight-junction” proteins.
 Image from Wikipedia
The tight junction proteins create a barrier between the inside of the gut (lumen) and the rest of the body to prevent harmful microorganisms passing into the blood, and also aid in the efficient uptake of nutrients.
So you can see from the diagram above that alterations in the mucus layer, or the formation of the impermeable barrier (going from “tight” to “leaky”) could have large health impacts including limiting proper absorption of nutrients and allowing “foreign invaders” into the bloodstream.
Image from Wikipedia
The tight junction proteins create a barrier between the inside of the gut (lumen) and the rest of the body to prevent harmful microorganisms passing into the blood, and also aid in the efficient uptake of nutrients.
So you can see from the diagram above that alterations in the mucus layer, or the formation of the impermeable barrier (going from “tight” to “leaky”) could have large health impacts including limiting proper absorption of nutrients and allowing “foreign invaders” into the bloodstream.
Zonulin and tight junctions
Enter zonulin. Zonulin was discovered in 2000 by researchers who were investigating the action of Vibrio cholerae (the bacteria which causes cholera) and its secreted zonula occludens toxin (ZOT). 3 ZOT is one of the toxins released by Vibrio cholerae which cause the severe diarrhea experienced by those with cholera, and acts by loosening the tight junctions of the gut. As these weaken, water can rapidly flow back into the gut putting sufferers at severe risk of dehydration. When studying this toxin, researchers identified that there was a human analogue which our own gut cells could release in order to regulate tight junction structure and function, which they named zonulin. 4 Importantly, zonulin remains the only modulator of intracellular tight junctions expressed so far that can affect gut function and health and associated immune response and so it is widely investigated.Zonulin in health and disease
So the first question researchers asked is, what diseases are associated with changes in zonulin activity or function?Celiac disease
As I discussed previously celiac disease describes a relatively common, in it affects approximately 1 in 135 people. Complications with diagnosis may mask the true number of celiac sufferers. 5 Celiac disease is a lifelong disorder of the digestive system, triggered by eating even trace amounts of wheat, where the intestine becomes inflamed and is unable to absorb nutrients properly, or process our food correctly. 5 You can see the effects of celiac disease on the lining of the gut below, the delicate villi and micro-villi are lost as the tissue has become inflamed.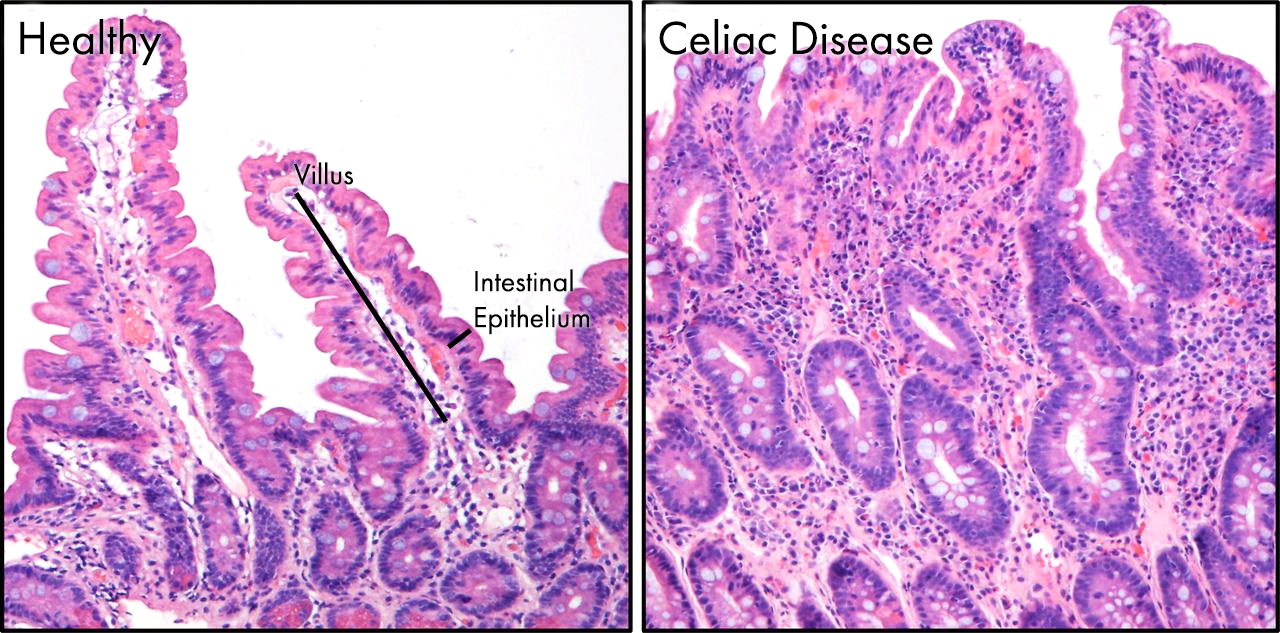 The villi seen in healthy gut are almost completely lost in celiac patients, greatly reducing the surface area and impairing nutrient absorption.
If it we look at another microscope picture of the gut from a healthy individual and one with celiac, this time stained for claudin (red) and ZO-1 (green) you can clearly see how the staining is much less intense and covers a far smaller area, suggesting breaks have appeared in the intestinal lining.
The villi seen in healthy gut are almost completely lost in celiac patients, greatly reducing the surface area and impairing nutrient absorption.
If it we look at another microscope picture of the gut from a healthy individual and one with celiac, this time stained for claudin (red) and ZO-1 (green) you can clearly see how the staining is much less intense and covers a far smaller area, suggesting breaks have appeared in the intestinal lining.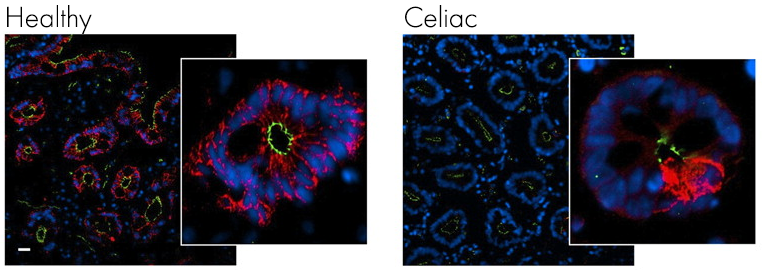 Image from Schumann M, et al. Gut. 20116
Looking at specific tight junction proteins claudin (red) and ZO-1 (green) the integrity of the gut epithelium is clearly compromised with fewer tight junction proteins present.
Serum zonulin has been shown to be significantly up-regulated in people with celiac disease compared to healthy control7, and also in the cells of the gut themselves when they were assessed after biopsy. 8
Image from Schumann M, et al. Gut. 20116
Looking at specific tight junction proteins claudin (red) and ZO-1 (green) the integrity of the gut epithelium is clearly compromised with fewer tight junction proteins present.
Serum zonulin has been shown to be significantly up-regulated in people with celiac disease compared to healthy control7, and also in the cells of the gut themselves when they were assessed after biopsy. 8
Type 1 diabetes
Type 1 diabetes (T1D) occurs when immune cells in the body attack and destroy beta cells in the pancreas leading to issues with insulin production and ultimately blood glucose control. Whilst some genetic markers for T1D have been described, the the causes of the remaining cases remains unknown. Image Link
The above image shows the pathway to the development of T1D.
A common feature of T1D are alterations in to the structure of the gut promoting a loss of tight junctions. These were typically described as symptoms of the disease9 (stage 3 or beyond in the above figure), however more recent studies have shown that loss of tight junctions actually occurs before disease onset (stage 1 or before in the above figure).10
This led scientists to suggest that alterations in gut permeability actually drive the development of T1D.1011 It is important to note here that you still have to be at genetic risk for T1D.
Several studies subsequently indirectly (hence the slightly lower science grade) linked increased zonulin with the onset of T1D following exposure to dietary triggers such as gliadin and glutenin the two main components of gluten from wheat.1213
Image Link
The above image shows the pathway to the development of T1D.
A common feature of T1D are alterations in to the structure of the gut promoting a loss of tight junctions. These were typically described as symptoms of the disease9 (stage 3 or beyond in the above figure), however more recent studies have shown that loss of tight junctions actually occurs before disease onset (stage 1 or before in the above figure).10
This led scientists to suggest that alterations in gut permeability actually drive the development of T1D.1011 It is important to note here that you still have to be at genetic risk for T1D.
Several studies subsequently indirectly (hence the slightly lower science grade) linked increased zonulin with the onset of T1D following exposure to dietary triggers such as gliadin and glutenin the two main components of gluten from wheat.1213




Hi,
I have read your article on Zonulin and the gut and I was looking to see if you are able to help me with some of my health symptoms? I became sick in 2011 with all over pain and stiffness with my arms and legs and it depends on the weather. My overactive thyroid became a problem after and was not under control with my medicine in which caused my goiter to grow to a least 3-4 pounds around my neck in which I needed to have a total thyroidectomy.They have done blood work which indicates that I have inflamation with my vesels. I had blood test for celiac disease in which I was told I do not have. I have been to so many specialist and primary and to a hollistic medicine Dr and she did blood work and said I have candida now and I have been trying to eat a gluten free diet and low carb and sugar intake diet. I have pain at the bottome of both my legs that feels like I have waits weighing me down and I am so crazy with information with all my doctors I have seen over the years I’m not sure what to think or believe when I try different treatments and no relief for my symtoms I suffer with daily. I was diagnosed with fibromyalgia but I feel I’m not getting properly diagnosed, do you think this is asscoiated with this Zonulin that can effect the gut?
If you can give me any information or recommendation of test or where to go with my health concern?
Thank You,
Jodie
Hi Aaron,
My teenage daughter is IGA deficient and is suffering from a gut dysbiosis which we have been managing. She was 90% better than she was seven months ago but recently has started to have pain and related issues again. She has seen several doctors ranging from a top rated university GI specialist to a Holistic MD to a Cognitive Behavioral Therapist. The holistic doctor has had the most success in treating her, however, I am interested in finding a functional medical doctor who specializes in GI issues but also has an understanding of IGA deficiency. With all of the research you have done, have you come across any that stand out in this area?
Excellent article. I was listening to Dr. Zach Bush explain how that his team has discovered that when the epithelial layer is exposed to gliadin & glyphosate (round up) at the same time, there is a catastrophic collapse of the tight junctions – far worse than exposure to either substance in the absence of the other.
His team believes that glyphosate up regulates the CXCR3 receptors, which bind to the gliadin triggering Zonulin to dissolve the tight junctions.
I came to the “www” looking for additional understanding of this process which is how I found your article. Thank you for writing & sharing.
Thanks Dave, glad you found it useful.
Unfortunately, I think there’s going to be a lot more stories coming out about the health impacts of widespread pest/herbicide usage. It’s going to be a difficult thing to balance…